Our goal is to preserve the structure of the entire brain at a fine ultrastructural level. This includes the synaptic architecture as well as detailed molecular information such as protein post-translational modifications, cellular epigenetic patterns, and subcellular distributions of molecules.
The reason we focus on preserving the brain alone, rather than the whole body, is that the brain is the only organ that cannot be damaged or replaced without fundamentally changing a person’s psychological identity. On the other hand, all other organs can be damaged or even replaced without affecting long-term memories or personality traits. Because the brain is irreplaceable and its preservation is challenging enough, we concentrate all of our efforts on it. This allows us to maximize the chances that we get this one essential task right.
The reason we focus on structural preservation is that we believe there is very convincing evidence that this is what is required for preserving the content of long-term memories and other aspects of long-term personal identity. Preserving a whole human brain with cellular viability intact is not yet possible, nor, in our view, close to being possible. But we believe preservation in a viable state is not necessary if the structure can be preserved well enough.
Whenever we can, we adopt the gold-standard methods for structural preservation from the peer-reviewed neuroscience literature. The methods used in any particular case are selected and adapted based on the condition of the patient and the practical circumstances. Our goal is always to preserve the greatest possible amount of structural information in the brain.
We are particularly informed by current best practices in neural electron microscopy, connectomics, neuropathology, and brain banking, which have been iteratively improved for more than a century to achieve high-quality structural preservation for clinical and research purposes.
When it comes to the structural preservation of an entire large brain, such as a human brain, preservation with aldehyde fixatives is widely considered by neuroscientists to be the best method. Decades of research have demonstrated that aldehyde-based chemical fixation can preserve the brain at a level of detail sufficient for electron microscopy, including the visualization of individual synapses and the spatial distribution of key molecules across cells.
Perfusion refers to driving the flow of solutions through the blood vessels to rapidly distribute preservation chemicals throughout a tissue. Perfusion of the brain can be performed with the brain inside or outside of the skull, and it can be performed via the cannulation of various different blood vessels.

By taking advantage of the existing vasculature, perfusion allows for the rapid delivery of fluids to the brain. It is well-established as the state-of-the-art method for preserving the entire brain, including human brains after moderately long periods of ischemia.
In human cases, we primarily cannulate the carotid and vertebral arteries for perfusion. In pet preservation cases, we primarily cannulate the aorta. In our hands, both of these methods appear to be effective at perfusing blood vessels throughout the brain.
It is important to note that perfusion becomes progressively more challenging as time passes after legal death. The longer the interval of ischemia, the less the vascular system permits the distribution of solution throughout the brain tissue. However, this is not an “all or none” switch. Instead, there is a gradual decline in perfusion efficacy as the mechanisms causing perfusion impairment, such as the degradation of endothelial cell tight junctions, accumulate.
If full perfusion is not likely to be possible, we may still attempt partial perfusion to accelerate the process of subsequent immersion fixation techniques and preserve as much structure as possible. The downside of attempting perfusion when the vasculature is compromised is primarily edema and associated tissue herniation. We monitor closely for edema and stop the procedure if it begins to cause significant brain swelling that could damage tissue structure.
When performing perfusion, we use formaldehyde-based solutions buffered at neutral pH levels. When the interval between legal death and perfusion is minimal, we add glutaraldehyde as well. Glutaraldehyde has a much faster crosslinking mechanism than formaldehyde, which means that it is particularly effective at preserving fine structural details. However, glutaraldehyde has poor tissue penetration compared to formaldehyde, which means it is not as useful for cases with delayed perfusion or when relying on immersion fixation. Moreover, there is a concern in the literature that the use of glutaraldehyde may decrease the speed of subsequent immersion fixation by creating a densely cross-linked outer layer that acts as a diffusion barrier.
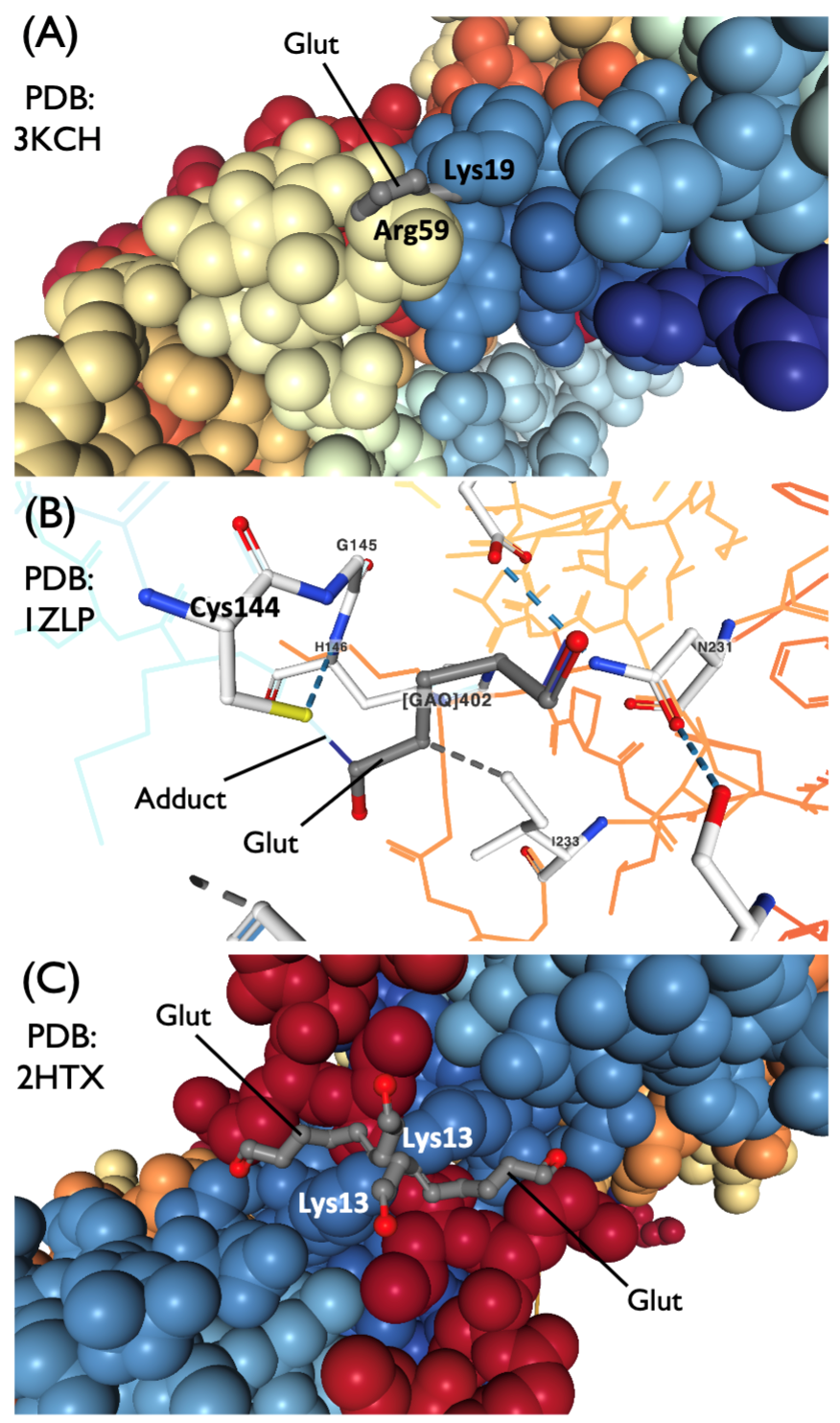
Examples of glutaraldehyde-protein crosslinking
From McKenzie 2019
With formaldehyde-based perfusion fixation alone, we have achieved excellent preservation quality as validated through electron microscopy in multiple brain regions. The preservation shows intact cellular membranes, clearly visible synapses with normal shape, and the ability to trace structures in 3d across image stacks.
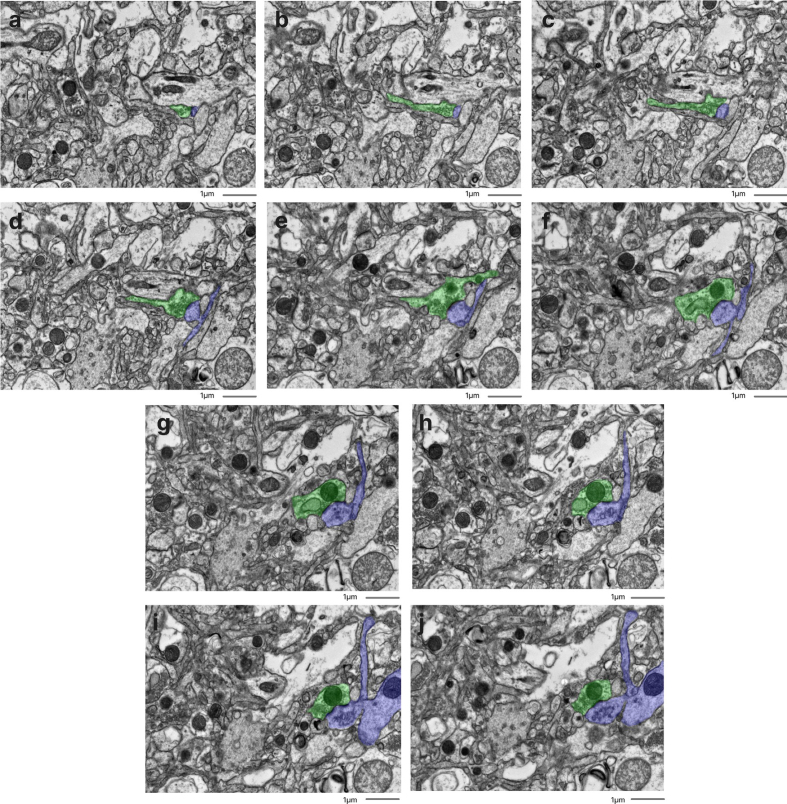
Example of synaptic connectivity tracing from a donated dog brain
From Garrood et al., 2025
It is important to point out that while we have achieved excellent ultrastructural preservation in selected samples taken from preserved brains, we have not yet been able to determine the conditions under which we can reliably preserve synaptic connectivity throughout all major regions across an entire human brain. That is a long-term research goal that we are working towards, which will require extensive validation studies using ultrastructural imaging techniques.
In cases where perfusion is not possible, we use immersion fixation as a backup method. In this method, after removing the brain from the skull, it is immediately submersed completely in aldehyde fixative solution. While immersion is substantially slower than perfusion to preserve the brain, some studies have shown that it can still achieve good preservation quality, particularly in cortical regions, which are believed to be important areas for long-term memory storage.
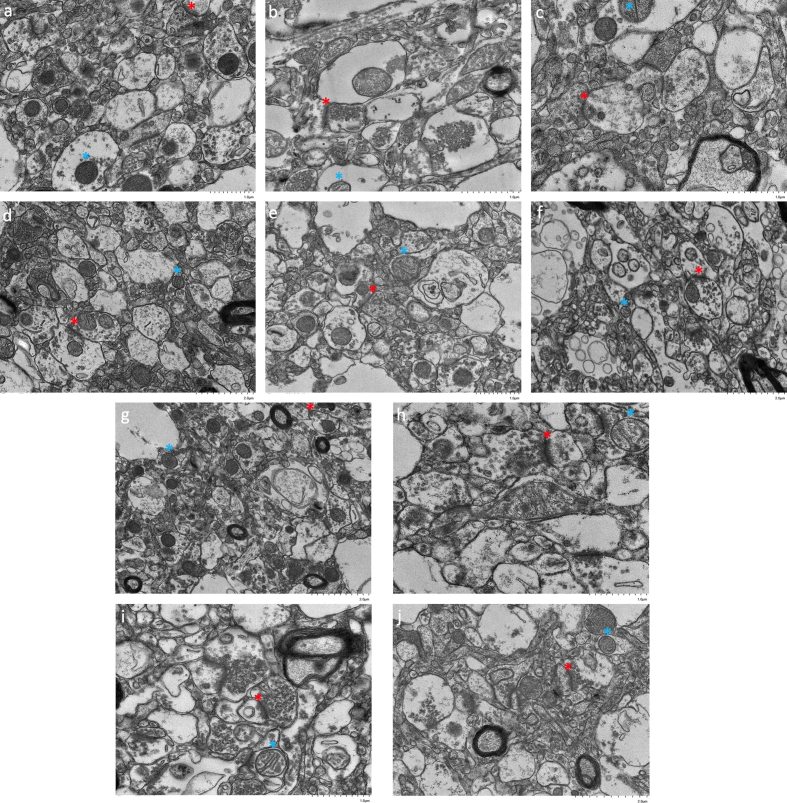
Examples of preservation quality in the cortex of immersion fixed brains, with various ischemic intervals
From Garrood et al., 2025
Currently we are storing preserved brains at refrigerator temperature (approximately 4°C) in aldehyde-containing preservation fluid. It is a remarkable and in our view underappreciated empirical finding that brains preserved in this manner have been found to have retention of neural circuitry for, at a minimum, decades. Mechanistically, it seems that aldehydes can crosslinks molecules to dramatically stabilize the native gel-like networks in cells and tissues. Multiple studies of human brain tissue stored this way for decades have shown clear preservation of cellular structure when examined with multiple types of imaging methods.
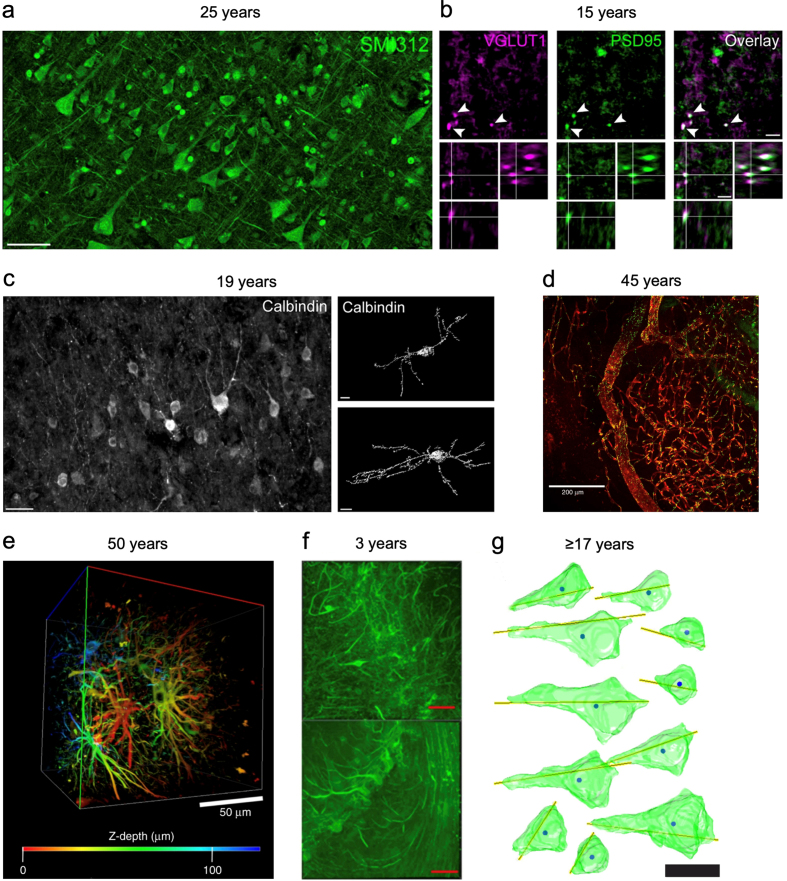
Examples of neural structure preservation after years of fluid preservation
From McKenzie et al., 2024
In the near future (likely within 1-3 years from now), we plan to switch to a cryopreservation protocol using subzero temperature storage. We are currently developing such a protocol, using a form of aldehyde-stabilized cryopreservation, where cryoprotectant agents would first diffuse into the brain and then it would be stored at -20°C. The plan is that most patients who are preserved now and stored at refrigerator temperature would be switched to this method. In the neuroscience literature, this is generally considered more stable for indefinite storage, and has been found to even prevent the loss of antigenicity for certain key epitopes after storage for decades (e.g., see Watson et al., 1986).
Our preservation approach is based on decades of neuroscience research showing that:
1. Long-term memories and personality are encoded in the brain’s physical structure, including in the patterns of synaptic connectivity.
2. Durable structures in the brain that seem to be important for memories, such as dendrites and synapses, are not immediately lost at the declaration of legal death, but rather degrade over the course of minutes to hours after the cessation of blood flow.
3. Aldehyde preservation can stabilize structures across the brain at the molecular level by cross-linking proteins and other biomolecules.
4. Properly preserved brain tissue can maintain its structure for decades when stored appropriately in the fluid state.
This forms the Scientific Basis of our approach for structural preservation.
Revival is certainly not possible today. It may become possible in the future with the development of Future Technology. At that point, revival could potentially be performed via whole brain emulation (also known as “mind uploading”) or biological repair (also known as molecular nanotechnology). Many people have preferences on this, and if so, you are free to express your preferences to us so that future people will be able to follow them. All we are currently focused on is preserving the information content in the brain to the best of our ability.
We take the assessment of preservation quality seriously. We employ multiple methods to validate our procedures, including gross examination and CT imaging. For patients participating in our research program, as well as whole body donors that are donated to science for research purposes only, we analyze small tissue samples using ultrastructural imaging to assess preservation quality at the subcellular level.
One of the challenges in the preservation field is the lack of systematic quality assessment and feedback. Historically, some preservation organizations have been reluctant to conduct rigorous research on their preservation methods because discovering limitations might suggest that past preservations were suboptimal. Additionally, acknowledging room for improvement is sometimes not considered good marketing. This is called the "No Feedback Problem."
We reject this approach. We freely admit that our methods have limitations and that we intend to improve them over time. While we believe our current protocols are the best available practices and that they may be sufficient to preserve the information encoding long-term memories and personal identity, we clearly cannot claim 100% certainty about this.
We regularly review our protocols based on the latest scientific literature and our own research findings. Every preservation teaches us something, and we incorporate these lessons into our evolving protocols. We believe this meliorist approach ultimately serves our patients better than claims of having already achieved perfect preservation.
Recommended Books:
The Future Loves You: How and Why We Should Abolish Death, by Ariel Zeleznikow-Johnston, PhD
Connectome: How the Brain's Wiring Makes Us Who We Are, by Sebastian Seung, PhD
Scientific Papers:
Krassner, M. M., Kauffman, J., Sowa, A., Cialowicz, K., Walsh, S., Farrell, K., Crary, J. F., & McKenzie, A. T. (2023). Postmortem changes in brain cell structure: A review. Free Neuropathology, 4, 10. doi.org/10.17879/freeneuropathology-2023-4790
McKenzie, A. T., Nnadi, O., Slagell, K. D., Thorn, E. L., Farrell, K., & Crary, J. F. (2024). Fluid preservation in brain banking: A review. Free Neuropathology, 5, 10. doi.org/10.17879/freeneuropathology-2024-5373
McKenzie, A. T., Zeleznikow-Johnston, A., Sparks, J. S., Nnadi, O., Smart, J., Wiley, K., Cerullo, M. A., de Wolf, A., Minerva, F., Risco, R., Church, G. M., de Magalhães, J. P., & Kendziorra, E. F. (2024). Structural brain preservation: A potential bridge to future medical technologies. Frontiers in Medical Technology, 6, 1400615. https://doi.org/10.3389/fmedt.2024.1400615
McKenzie, A. T., Wowk, B., Arkhipov, A., Wróbel, B., Cheng, N., & Kendziorra, E. F. (2024). Biostasis: A roadmap for research in preservation and potential revival of humans. Brain Sciences, 14(9), 942. https://doi.org/10.3390/brainsci14090942
Zeleznikow-Johnston, A., Kendziorra, E. F., & McKenzie, A. T. (2025). What are memories made of? A survey of neuroscientists on the structural basis of long-term memory. PloS One, 20(6), e0326920. doi.org/10.1371/journal.pone.0326920
McKenzie, A. T., Cerullo, M., Farahani, N., Sparks, J. S., Londoño, T., de Wolf, A., Dziennis, S., Wróbel, B., German, A., Kendziorra, E. F., de Magalhães, J. P., Cho, W., Perry, R. M., & More, M. (2025). Practitioner forecasts of technological progress in biostasis. ArXiv:2507.17274 [q-Bio:NC]. http://arxiv.org/abs/2507.17274